
Problem PageQuestion Liquid octane will react with gaseous oxygen to produce gaseous carbon dioxide and gaseous water . Suppose 10. g of octane is mixed with 61.9 g of oxygen. Calculate the minimum mass of octane that could be left over by the chemical reaction. Round your answer to significant digits.

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 02:00, webbhlharryteach
In which of these cases are the two wave points considered to be in phase with each other?
Answers: 1

Chemistry, 22.06.2019 02:30, ethanmel21
Which compound contains both ionic and covalent bonds? a) hbr b)cbr4 c)nabr d) naoh
Answers: 2


Chemistry, 22.06.2019 14:30, darkghostmist
What type of reaction fuels the processes seen here?
Answers: 2
Do you know the correct answer?
Problem PageQuestion Liquid octane will react with gaseous oxygen to produce gaseous carbon dioxide...
Questions in other subjects:

Mathematics, 11.03.2021 23:00


Physics, 11.03.2021 23:00




Mathematics, 11.03.2021 23:00

Mathematics, 11.03.2021 23:00

Mathematics, 11.03.2021 23:00

Mathematics, 11.03.2021 23:00


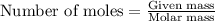


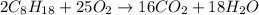
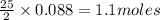 of ethane
of ethane



