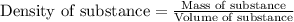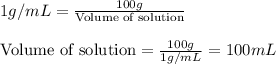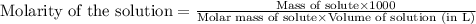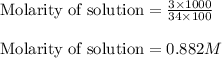Hydrogen peroxide, a disinfectant, contains 3.0% (w/w) hydrogen peroxide in water. This means there are 3.0 grams of hydrogen peroxide in every 100. grams of solution. Assuming this solution has a density of 1.00 g/mL, what is the molar concentration of this solution

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 18:30, mimithurmond03
A6.10 m nacl can be made by adding [x]g of nacl to a container and making the volume of water up to the 1.00 l line
Answers: 1

Chemistry, 22.06.2019 05:40, wanderer3653
Fill in the coefficients that will balance the following reaction: a0cr2(so4)3 + a1agno3
Answers: 3

Chemistry, 22.06.2019 16:10, 00015746
Predict the reactants of this chemical reaction. that is, fill in the left side of the chemical equation. be sure the equation you submit is balanced. (you can edit both sides of the equation to balance it, if you need to.) note: you are writing the molecular, and not the net ionic equation. > cacl2(aq) + h20(l)
Answers: 2
Do you know the correct answer?
Hydrogen peroxide, a disinfectant, contains 3.0% (w/w) hydrogen peroxide in water. This means there...
Questions in other subjects: