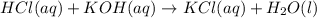During a laboratory activity, a student places 25.0 mL of HCl(aq) of unknown concentration into a flask. The student adds four drops of phenolphthalein to the solution in the flask. The solution is titrated with 0.150 M KOH(aq) until the solution appears faint pink. The volume of KOH(aq) added is 18.5 mL. Complete the equation for the neutralization reaction that occurs during the titration: KOH (aq) + HCl (aq) -->

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 06:00, momof7hardings
When would a bouncy ball have the most potential energy
Answers: 2

Chemistry, 22.06.2019 08:30, audrey1256
Which change in temperature is the smallest? a change of 1 thomson degree a change of 1 kelvin degree a change of 1 fahrenheit degree a change of 1 celsius degree
Answers: 1

Do you know the correct answer?
During a laboratory activity, a student places 25.0 mL of HCl(aq) of unknown concentration into a fl...
Questions in other subjects:









History, 22.04.2021 02:20