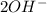What information will you be given in this experiment that will allow you to calculate the moles of hydroxide produced by dissolving calcium hydroxide in water? 1. Milligrams of Ca 2. Molar mass of hydroxide 3. Initial Volume of HCl 4. Molar mass of HCl 5. Final Volume of HCl 6. Molarity of HCl

Answers: 1
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 06:30, angelrenee2000
Ineed someone to see if my answers are correct! if any are wrong let me know what the correct answers would be and how to get that answer! 1. how many moles of sodium chloride are in 28 grams od nacl? a. 265 mole naclb. 856 mole naclc. 479 mole of nacld. 1.2 mole nacl < my choice2. 734 grams of lithium sulfate (li2so4) are dissolved to make 2500 ml of solution what is rhe molaratiy? a. 2.67 mb. 4.56 mc. 3.89 m < my choiced. 1.78 m3. how many grams of cacl2 would be dissolved in 3.0 l of a 0.50 m solution of cacl2? a. 250 g cacl2 b. 166.5 g cacl2c. 113.65 g cacl2d. 98 g cacl2 < my choice4. suppose you had 58.44 g of nacl and you dissolved it in exactly 2.00 liters. the molarity if the solution would be 0.5 mtrue < my choicefalse 5. i would need 22g of naoh to make a 3.0 m solution using 250 ml of solvent. true < my choicefalse6. identify the solute: you have a .0195 m solution made from using 6.5 g of solute and 3 l of solvent. identify the solute by solving for molar weight. a. the solute is nacl because the molar weight is 58.43 g/mol < my choiceb. the solute is h2so4 because the molar weight is 98.06 g/molc. the solute is cacl2 because the molar weight is 111.11 g/mol
Answers: 1

Do you know the correct answer?
What information will you be given in this experiment that will allow you to calculate the moles of...
Questions in other subjects:

Mathematics, 09.07.2019 02:00

World Languages, 09.07.2019 02:00

Mathematics, 09.07.2019 02:00

Business, 09.07.2019 02:00


History, 09.07.2019 02:00


Geography, 09.07.2019 02:00

Mathematics, 09.07.2019 02:00

History, 09.07.2019 02:00


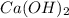 →
→ 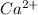 +
+