
Chemistry, 26.03.2020 22:01, kharmaculpepper
Given the following balanced equation, determine the rate of reaction with respect to [Cl2]. If the rate of disappearance of Cl2 is 4.44 × 10-2 M/s, what is the rate of disappearance of NO? 2 NO(g) + Cl2(g) → 2 NOCl(g) Given the following balanced equation, determine the rate of reaction with respect to [Cl2]. If the rate of disappearance of Cl2 is 4.44 × 10-2 M/s, what is the rate of disappearance of NO? 2 NO(g) + Cl2(g) → 2 NOCl(g) 2.22 × 10-2 M/s 1.11 × 10-1 M/s 4.44 × 10-2 M/s 8.88 × 10-2 M/s 5.25 × 10-2 M/s

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 13:00, devontemiles8868
One of the hopes for solving the world's energy problem is to make use of the fusion reaction 21h +31h --> 42he + 10n + energy how much energy is released when 1 mol of deuterium is fused with 1 mol of tritium according to the above reaction? the masses of the atoms and the neutrons are as follows: 21h = 2.0140 amu 31h = 3.01605 amu 42he = 4.002603 amu 10n = 1.008665 amu. the speed of light is 2.9979 x 108 m/s.
Answers: 1

Chemistry, 22.06.2019 13:30, bryce99
In a ni-cd battery, a fully charged cell is composed of nickelic hydroxide. nickel is an element that has multiple oxidation states. assume the following proportions of the states: nickel charge proportions found 0 0.17 +2 0.3 +3 0.33 +4 0.5 (a) determine the mean of the nickel charge. enter the answer to 2 decimal places.(b) determine the cumulative distribution function of nickel charge.
Answers: 2


Chemistry, 22.06.2019 22:30, eduardoguizar8787
Which one of the following bonds would you expect to be the most polar? a) b–h b) n–h c) p–h d) al–h e) c–h
Answers: 1
Do you know the correct answer?
Given the following balanced equation, determine the rate of reaction with respect to [Cl2]. If the...
Questions in other subjects:

Mathematics, 17.09.2019 21:50

Biology, 17.09.2019 21:50

Mathematics, 17.09.2019 21:50


History, 17.09.2019 21:50






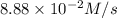
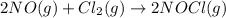
![-\frac{1d[NO]}{2dt}=-\frac{d[Cl_2]}{dt}=+\frac{1d[NOCl]}{2dt}](/tpl/images/0565/9272/04d4d.png)
![\frac{-d[Cl_2]}{dt}]=4.44\times 10^{-2}M/s](/tpl/images/0565/9272/271c7.png)
![-\frac{1d[NO]}{dt}=2\times {\frac{-d[Cl_2]}{dt}=2\times 4.44\times 10^{-2}M/s=8.88\times 10^{-2}M/s](/tpl/images/0565/9272/dea34.png)




