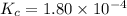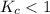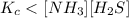Chemistry, 26.03.2020 21:33, demienarravo
The equilibrium constant, Kc, for the following reaction is 1.80×10-4 at 298 K. NH4HS(s) NH3(g) + H2S(g) This reaction is Reactant favored at equilibrium. Enter PRODUCT or REACTANT. The concentrations of NH3 and H2S will be at equilibrium. Enter HIGH or LOW.

Answers: 3
Other questions on the subject: Chemistry


Chemistry, 23.06.2019 09:30, MendesArmy333
Northern was a learned a fairly cold climate caused by see one from the atlantic ocean, but se was real and tends to be much warmer, sonia look good causes difference. a. cool wins cannot blow across a leg into the south mountains, b. prevent cold air from blowing over into south sea, c.the south is at much higher elevation, so it is closer to the sun, d. suppose early and has a sperience a drastic climate change in the past few years.
Answers: 1

Chemistry, 23.06.2019 12:30, dependentclause5828
Idid a lab for chemistry where we put nails in a copper (ii) chloride solution. 1. why did the reaction stop? which reactant was used up? how do you know? 2. describe what was happening to the atoms of iron and copper during the reaction. what is this type of reaction called? 3. what would happen to the ratio of copper to iron if you had placed more nails in the beaker? if you had let the reaction go for less time? 4. what is the accepted ratio of copper atoms to iron atoms in this reaction? account for differences between your experimental value and the accepted value. write the balanced equation for the reaction.
Answers: 2

Chemistry, 23.06.2019 16:30, firstone04kr
Aresearcher wants to experiment with an element that reacts like phosphorus (p) but has a greater atomic mass. which element should the researcher select for the experiment?
Answers: 1
Do you know the correct answer?
The equilibrium constant, Kc, for the following reaction is 1.80×10-4 at 298 K. NH4HS(s) NH3(g) + H2...
Questions in other subjects:

Mathematics, 06.04.2020 10:20

Social Studies, 06.04.2020 10:20



Biology, 06.04.2020 10:23


Mathematics, 06.04.2020 10:26

English, 06.04.2020 10:26

Biology, 06.04.2020 10:26

Chemistry, 06.04.2020 10:26


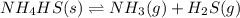
![K_c=[NH_3][H_2S]](/tpl/images/0565/8253/ff5ed.png)
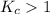 ; the reaction is product favored.
; the reaction is product favored.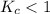 ; the reaction is reactant favored.
; the reaction is reactant favored.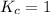 ; the reaction is in equilibrium.
; the reaction is in equilibrium.