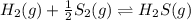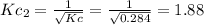Chemistry, 26.03.2020 21:03, estefaniapenalo
When heated, hydrogen sulfide gas decomposes according to the equation: 2H2S(g) → 2H2(g) + 2S2(g) A 6.75 gram sample of H2S(g) is introduced into an evacuated rigid 0.75 L container. The sealed container is heated to 283 K and 6.42 x 10 ^–2 mol of S2 gas is present at equilibrium.
a. Calculate the equilibrium concentration, in mol/L, of the H2(g) in the container at 283 K.
b. Calculate the equilibrium concentration, in mol/L, of the H2S(g) in the container at 283 K.
c. Calculate the value of the equilibrium constant, Kc, for the decomposition reaction at 283 K.
d. Calculate the partial pressure of S2(g) in atm in the container at equilibrium at 283 K.
e. Calculate the value of the equilibrium constant, Kc, for the reaction
H2(g) + 1/2 S2(g) → H2S (g) at 283 K.

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 18:10, gonzalesalexiaouv1bg
Using complete sentences, explain how to predict the products and balance the reaction between sulfuric acid and potassium hydroxide.
Answers: 1

Chemistry, 21.06.2019 20:30, mvtthewisdead
If 10.g of agno3 is available, what volume of 0.25 m agno3 can be prepared
Answers: 1


Chemistry, 22.06.2019 12:30, skaterwolf1317
Which statement is true about this reaction? 14n+1h 15o it is a practical source of energy on earth. it occurs only outside the solar system. its product is heavier than each of its reactants. it shows the critical mass of an element.
Answers: 2
Do you know the correct answer?
When heated, hydrogen sulfide gas decomposes according to the equation: 2H2S(g) → 2H2(g) + 2S2(g) A...
Questions in other subjects:





Mathematics, 08.04.2022 20:00

Social Studies, 08.04.2022 20:00


Advanced Placement (AP), 08.04.2022 20:30



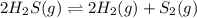
 due to stoichiometry and the reaction extent, turns out:
due to stoichiometry and the reaction extent, turns out:![K=\frac{[H_2]_{eq}^2[S_2]_{eq}}{[H_2S]_{eq}^2}](/tpl/images/0565/7677/a5ff5.png)
![[H_2S]_0=\frac{6.75g/(34g/mol)}{0.75L} =0.265M](/tpl/images/0565/7677/26ae9.png)
![[S_2]_{eq}=x=\frac{6.42x10^{-2}mol}{0.75L}=0.0856M](/tpl/images/0565/7677/c4de0.png)
![[H_2]_{eq}=2x=2*0.0856M=0.171M](/tpl/images/0565/7677/0a782.png)
![[H_2S]_{eq}=0.265M-2x=0.265M-2*0.0856M=0.0938M](/tpl/images/0565/7677/de91b.png)
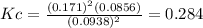
![p_{S_2}=[S_2]RT= 0.0856\frac{mol}{L}*0.082\frac{atm*L}{mol*K}*283K=1.99atm](/tpl/images/0565/7677/136fa.png)