
Chemistry, 26.03.2020 20:48, rubycarbajal
The explosive nitroglycerin (C3H5N3O9) decomposes rapidly upon ignition or sudden impact according to the following balanced equation:
4C3H5N3O9(l)→12CO2(g)+10H2O(g)+6N2( g)+O2(g) ΔH∘rxn=−5678kJ
Required:
Calculate the standard enthalpy of formation (ΔH∘f) for nitroglycerin.

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 16:50, mathiscool7
Ajet plane is speeding down the runway during takeoff. air resistance is not negligible. identify the forces on the jet.
Answers: 3


Chemistry, 23.06.2019 10:30, fatheadd2007
When a chemist collects hydrogen gas over water, she ends up with a mixture of hydrogen and water vapor in her collecting bottle if the pressure in the collecting bottle is 97.1 kilopascals and the vapor pressure of the water is 3 2 kilopascals, what is the partial pressure of the hydrogen?
Answers: 1
Do you know the correct answer?
The explosive nitroglycerin (C3H5N3O9) decomposes rapidly upon ignition or sudden impact according t...
Questions in other subjects:




Physics, 06.07.2019 06:00

Mathematics, 06.07.2019 06:00


Physics, 06.07.2019 06:00


Mathematics, 06.07.2019 06:00


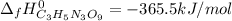
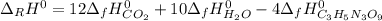
![\Delta _fH^0_{C_3H_5N_3O_9}=\frac{1}{4} (12\Delta _fH^0_{CO_2}+10\Delta _fH^0_{H_2O}-\Delta _RH^0)\\\Delta _fH^0_{C_3H_5N_3O_9}=\frac{1}{4mol} [12(-393.5kJ)+10(-241.8kJ)-(-5678kJ)]\\\Delta _fH^0_{C_3H_5N_3O_9}=-365.5kJ/mol](/tpl/images/0565/7176/fdf74.png)




