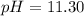Chemistry, 26.03.2020 20:36, gennhill14
A chemist titrates 100.0mL of a 0.3444M hydrocyanic acid HCN solution with 0.8414M KOH solution at 25°C . Calculate the pH at equivalence. The pKa of hydrocyanic acid is 9.21 . Round your answer to 2 decimal places.

Answers: 3
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 04:30, EinsteinBro
Which statement best describes the relationship between period and frequency of light waves? a) in wave b the period increases and the frequency decreases from wave a. b) in wave a the period increases and the frequency decreases from wave b. c) in wave b the period is shorter and the frequency is greater than in wave a. d) in wave a the period is shorter and the frequency is greater than in wave b.
Answers: 1

Chemistry, 22.06.2019 06:00, coopera1744
Find the mass in grams of 1.37x1020 particles of h3po4
Answers: 2
Do you know the correct answer?
A chemist titrates 100.0mL of a 0.3444M hydrocyanic acid HCN solution with 0.8414M KOH solution at 2...
Questions in other subjects:

Geography, 27.06.2019 00:00

History, 27.06.2019 00:00

Mathematics, 27.06.2019 00:00


Biology, 27.06.2019 00:00

Mathematics, 27.06.2019 00:00

Geography, 27.06.2019 00:00



English, 27.06.2019 00:00


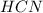 = 0.3444 M
= 0.3444 M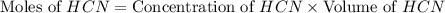
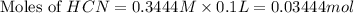
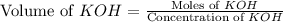
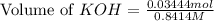
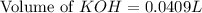
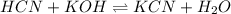
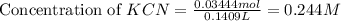
![pH=\frac{1}{2}[pK_w+pK_a+\log C]](/tpl/images/0565/6867/b44e5.png)
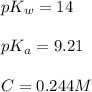
![pH=\frac{1}{2}[14+9.21+\log (0.244)]](/tpl/images/0565/6867/e6178.png)