
Chemistry, 26.03.2020 20:31, sparky1234
1. Tooth enamel consists mainly of hydroxyapatite (Ca5(PO4)3(OH). When tin(II)fluoride is added to toothpaste it reacts with the enamel to produce a more decay-resistant material fluoroapatite ( Ca5(PO4)3F. The by-products of this reaction are tin(II)oxide and water. What mass of hydroxyapatite can be converted to fluoroapatite by reaction with 0.115 grams of tin(II)fluoride

Answers: 1
Other questions on the subject: Chemistry



Chemistry, 22.06.2019 16:10, 00015746
Predict the reactants of this chemical reaction. that is, fill in the left side of the chemical equation. be sure the equation you submit is balanced. (you can edit both sides of the equation to balance it, if you need to.) note: you are writing the molecular, and not the net ionic equation. > cacl2(aq) + h20(l)
Answers: 2

Chemistry, 22.06.2019 17:20, phanuel642
The small bags of silica gel you often see in a new shoe box are placed there to control humidity. despite its name, silica gel is a solid. it is a chemically inert, highly porous, amorphous form of sio2. because water vapor readily adsorbs onto the surface of silica gel, it acts as a desiccant. despite not knowing mechanistic details of the adsorption of water onto silica gel, from the information provided you should be able to make an educated guess about the thermodynamic characteristics of the process. predict the signs for δg, δh, and δs for the adsorption of water.
Answers: 2
Do you know the correct answer?
1. Tooth enamel consists mainly of hydroxyapatite (Ca5(PO4)3(OH). When tin(II)fluoride is added to t...
Questions in other subjects:



English, 27.04.2021 04:20



Mathematics, 27.04.2021 04:20

Mathematics, 27.04.2021 04:20

History, 27.04.2021 04:20

Chemistry, 27.04.2021 04:20


 .....(1)
.....(1)
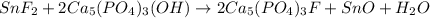
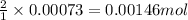 of hydroxyapatite
of hydroxyapatite 




