
Chemistry, 26.03.2020 20:02, kaleahlove13
Name: Date: Period: 3/23 - 3/27 Assignment 1: Gas Law Practice Problems 1. A gas occupies 12.3 liters at a pressure of 40.0 mm Hg. What is the volume when the pressure is increased to 60.0 mm Hg? Given: Equation: 2. A gas occupies 900.0 mL at a temperature of 27.0 °C. What is the volume at 132.0 °C? Given: Equation: 3. If a gas in a closed container is pressurized from 15.0 atmospheres to 16.0 atmospheres and its original temperature was 25.0 °C, what would the final temperature of the gas be? Given: Equation: 4. A sample of gas occupies 2.00 l with 5.00 moles present. What would happen to the volume if the number of moles is increased to 10.0? Given: Equation:

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 06:00, hdjsjfjruejchhehd
The tilt of the earth's axis of rotation is responsible for the a) ocean's tides. b) size of the moon. c) brightness of stars. d) earth’s seasons.
Answers: 1


Chemistry, 22.06.2019 12:30, ethanw8973
If 22.5 liters of oxygen reacted with excess of hydrogen, how many liters of water vapor could be produced?
Answers: 3
Do you know the correct answer?
Name: Date: Period: 3/23 - 3/27 Assignment 1: Gas Law Practice Problems 1. A gas occupies 12.3 liter...
Questions in other subjects:


Mathematics, 10.10.2019 12:30




Geography, 10.10.2019 12:30


Mathematics, 10.10.2019 12:30


Mathematics, 10.10.2019 12:30


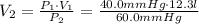 = 8.2 l
= 8.2 l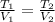
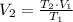 =
= 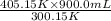 = 1214.84 ml
= 1214.84 ml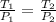
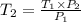
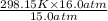 = 318.027 K
= 318.027 K



