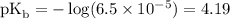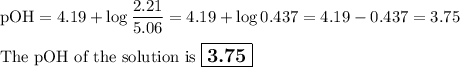Chemistry, 26.03.2020 03:19, danielle413
Calculate the pOH of a solution that results from mixing 46 mL of 0.11 M trimethylamine ((CH3)3N) with 17 mL of 0.13 M (CH3)3NHCl. The Kb value for (CH3)3N is 6.5 x 10-5.

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 16:30, shreyapatel2004
How many atoms of oxygen are contained in 160 grams of n2o3
Answers: 2

Chemistry, 21.06.2019 23:30, ashleyjaslin
Calculate the expected ph values of the buffer systems from the experiments (a, b,c, d), using the henderson- hasselbalch equation, ph-pka+log[a-]/[ha]. use for pka values carbonic acid= 6.37, and acetic acid= 4.75.
Answers: 2

Chemistry, 22.06.2019 09:40, loveoneonly9153
Consider this initial-rate data at a certain temperature for the reaction described by
Answers: 1

Chemistry, 22.06.2019 18:30, ashleymer384
Two people each hold the end of a rope and create waves by moving their arms up and down. this wave is best classified as a transverse wave because a) both the rope particles and the wave are moving in the same direction. b) the wave is moving up and down as the particles of the rope move horizontally. c) the wave is moving horizontally as the particles of the rope move up and down. eliminate d) the wave is moving in a parallel direction with the motion of the person's arms.
Answers: 3
Do you know the correct answer?
Calculate the pOH of a solution that results from mixing 46 mL of 0.11 M trimethylamine ((CH3)3N) wi...
Questions in other subjects:

Mathematics, 26.08.2020 07:01


Mathematics, 26.08.2020 07:01


Mathematics, 26.08.2020 07:01


Mathematics, 26.08.2020 07:01



Biology, 26.08.2020 07:01



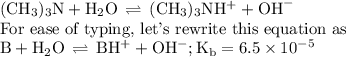
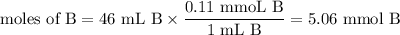
![\text{pOH} = \text{p}K_{\text{b}} + \log\dfrac{[\text{BH}^{+}]}{\text{[B]}}](/tpl/images/0564/6217/7ee6a.png)