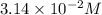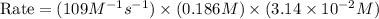Chemistry, 25.03.2020 23:55, markusovaevelyn532
The reaction of nitrogen monoxide with ozone at 25 oC NO + O3NO2 + O2 is first order in NO and first order in O3. Complete the rate law for this reaction in the box below. Use the form k[A]m[B]n... , where '1' is understood for m, n ... (don't enter 1) and concentrations taken to the zero power do not appear. Rate =
In an experiment to determine the rate law, the rate constant was determined to be 109 M-1s-1. Using this value for the rate constant, the rate of the reaction when [NO] = 0.186 M and [O3] = 3.14×10-2 M would be [blank] Ms-1.

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 05:50, vanessa051266
In an exothermic reaction the bonding energy of the product is: less than the reactants same as the reactants greater than the reactants dependent upon the presence of a catalyst
Answers: 1

Chemistry, 22.06.2019 09:40, cheesecake1919
Which diagram shows the correct way to represent an ionic compound of magnesium oxide?
Answers: 3


Chemistry, 23.06.2019 01:30, babygirl091502
In what way do investigations build scientific knowledge? the results of investigations lead to questions that cannot be tested. they reflect the opinions and social values of scientists, ensuring valid information. the results of investigations lead to new questions, which lead to new investigations. they are not influenced by the research of earlier scientists, so they are able to address gaps in understanding. i
Answers: 1
Do you know the correct answer?
The reaction of nitrogen monoxide with ozone at 25 oC NO + O3NO2 + O2 is first order in NO and first...
Questions in other subjects:

English, 13.12.2021 05:10

Mathematics, 13.12.2021 05:10

English, 13.12.2021 05:10



Social Studies, 13.12.2021 05:10

Mathematics, 13.12.2021 05:10

Chemistry, 13.12.2021 05:10


Health, 13.12.2021 05:10


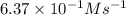
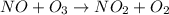
![\text{Rate}=k[NO]^a[O_3]^b](/tpl/images/0564/1543/2796d.png)
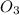 = 1
= 1![\text{Rate}=k[NO]^1[O_3]^1](/tpl/images/0564/1543/85a0b.png)
![\text{Rate}=k[NO][O_3]](/tpl/images/0564/1543/1a568.png)
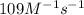
![[O_3]](/tpl/images/0564/1543/8b13e.png) = concentration of
= concentration of