
Chemistry, 25.03.2020 23:39, sjjsksksj1590
At equilibrium, a 4.50 L container has 2.6 g of carbon, CO2 at a partial pressure of 0.0020 atm, and a total pressure of 0.572 atm. Calculate KP for this reaction at 725oC.

Answers: 3
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 20:30, Schoolworkspace453
Consider the following unbalanced equation for the combustion of hexane: αc6h14(g)+βo2(g)→γco2(g)+δh2o(g) part a balance the equation. give your answer as an ordered set of numbers α, β, γ, use the least possible integers for the coefficients. α α , β, γ, δ = nothing request answer part b determine how many moles of o2 are required to react completely with 5.6 moles c6h14. express your answer using two significant figures. n n = nothing mol request answer provide feedback
Answers: 2

Chemistry, 22.06.2019 22:00, shaylasimonds587
The volume of an unknown substance in a sealed glass jar is 50 milliliters. the volume of the jar is 200 milliliters. which state of matter could the substance be?
Answers: 2

Chemistry, 23.06.2019 06:00, girlwonder326
+= + + balance the equation on coefficients, ex. 1,1,1
Answers: 2
Do you know the correct answer?
At equilibrium, a 4.50 L container has 2.6 g of carbon, CO2 at a partial pressure of 0.0020 atm, and...
Questions in other subjects:



Mathematics, 26.10.2021 18:10


Mathematics, 26.10.2021 18:10


Biology, 26.10.2021 18:10




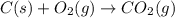
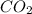 = 0.0020 atm
= 0.0020 atm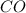 = Total pressure at equilibrium - Equilibrium pressure of
= Total pressure at equilibrium - Equilibrium pressure of 
![K_p=\frac{[p_{CO}]^2}{[p_{CO_2}}](/tpl/images/0564/1099/e1319.png)
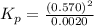
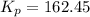
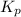 for this reaction at
for this reaction at  is 162.45
is 162.45




