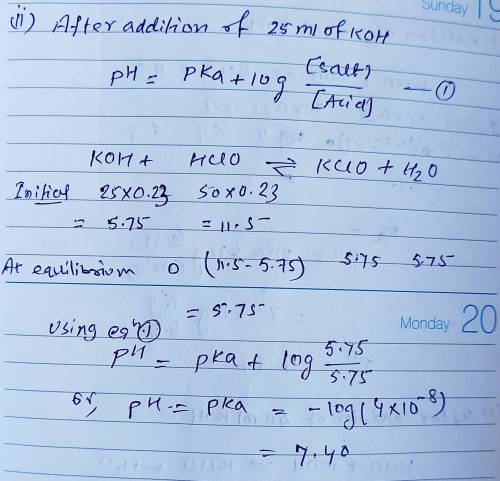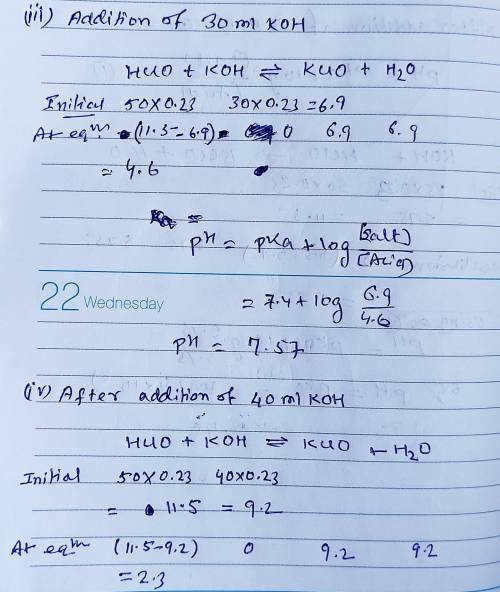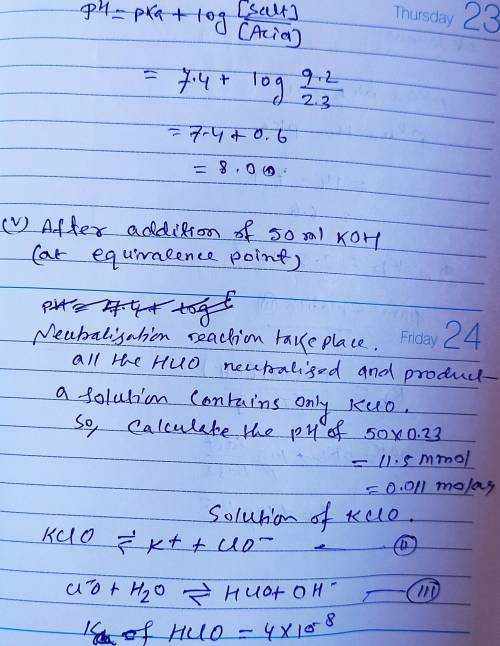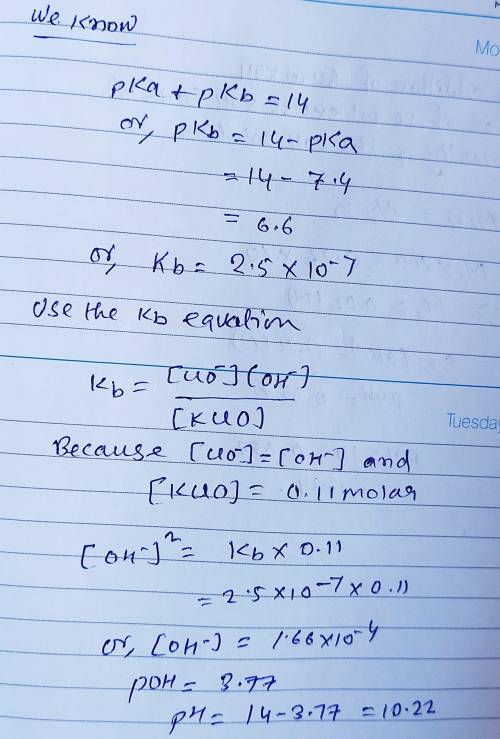Chemistry, 25.03.2020 22:30, eshaesmot12345
Calculate the pH for each case in the titration of 50.0 mL of 0.230 M HClO ( aq ) 0.230 M HClO(aq) with 0.230 M KOH ( aq ) . 0.230 M KOH(aq). Use the ionization constant for HClO . HClO. What is the pH before addition of any KOH ? KOH? pH = pH= What is the pH after addition of 25.0 mL KOH ? 25.0 mL KOH? pH = pH= What is the pH after addition of 30.0 mL KOH ? 30.0 mL KOH? pH = pH= What is the pH after addition of 50.0 mL KOH ? 50.0 mL KOH? pH = pH= What is the pH after addition of 60.0 mL KOH ? 60.0 mL KOH? pH =

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 04:30, only1cache
When the water vapor cools it condenses select a number that represents his process on the
Answers: 3

Chemistry, 22.06.2019 17:30, Naysa150724
Oil rich countries in the middle east cover about 4% of earths total land area but prossess about 48% of the worlds known oil reserves what is the main reason for high concentration of reserves in this part of the world
Answers: 3
Do you know the correct answer?
Calculate the pH for each case in the titration of 50.0 mL of 0.230 M HClO ( aq ) 0.230 M HClO(aq) w...
Questions in other subjects:





Biology, 25.12.2019 03:31