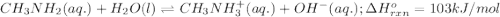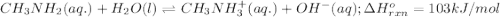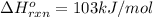Chemistry, 25.03.2020 22:37, kamjay2006
Use Le Châtelier's principle to predict how the equilibrium for the weak base methylamine responds to the indicated changes. CH 3 NH 2 ( aq ) + H 2 O ( l ) − ⇀ ↽ − CH 3 NH + 3 ( aq ) + OH − ( aq ) Δ H ∘ rxn = 103 kJ / mol

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 14:00, IdkHowToDoMath
What term describes technology that operates on an atomic level
Answers: 2

Chemistry, 22.06.2019 21:20, paatnguyyen
One way in which the useful metal copper is produced is by dissolving the mineral azurite, which contains copper(ii) carbonate, in concentrated sulfuric acid. the sulfuric acid reacts with the copper(ii) carbonate to produce a blue solution of copper(ii) sulfate. scrap iron is then added to this solution, and pure copper metal precipitates out because of the following chemical reaction: (s) (aq) (s) (aq) suppose an industrial quality-control chemist analyzes a sample from a copper processing plant in the following way. he adds powdered iron to a copper(ii) sulfate sample from the plant until no more copper will precipitate. he then washes, dries, and weighs the precipitate, and finds that it has a mass of .
Answers: 2

Chemistry, 23.06.2019 08:40, Riplilpeep
Which statement is true according to the kinetic theory? a. molecules of different gases with the same mass and temperature always have the same average density. b. molecules of different gases with the same mass and temperature always have the same average volume. c. molecules of different gases with the same mass and temperature always have the same pressure. d. molecules of different gases with the same mass and temperature always have the same molecular mass. e. molecules of different gases with the same mass and temperature always have the same average kinetic energy.
Answers: 1

Chemistry, 23.06.2019 12:30, okasiafolk27
15) a substance used in manufacturing gasoline consists of finely divided platinum supported on an inert solid. suppose that the platinum is formed by the high temperature reaction between platinum (iv) oxide and hydrogen gas. the other product is water. a) write and balance the equation b) how many grams of hydrogen are needed to produce 1.0 g of platinum metal? c) how many moles of water are produced at the same time? how many grams? ( show work, .)
Answers: 1
Do you know the correct answer?
Use Le Châtelier's principle to predict how the equilibrium for the weak base methylamine responds t...
Questions in other subjects:

Mathematics, 15.01.2021 01:40

Mathematics, 15.01.2021 01:40

Physics, 15.01.2021 01:40





Mathematics, 15.01.2021 01:40

Mathematics, 15.01.2021 01:40

Social Studies, 15.01.2021 01:40