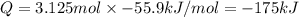125 grams of solid sodium hydroxide is combined with an excess of aqueous hydrochloric acid. The heat of neutralization for the formation of water -55.9 kJ/mole. The heat of solvation (it might be called the heat of solution) for sodium hydroxide is 41.0 kJ/mole What quantity of heat is released?

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 20:30, mimithurmond03
The speed of light is around 6.706×10^8 miles per hour. what is the speed of light in units of miles per minute?
Answers: 2

Chemistry, 22.06.2019 01:30, montoyaricardo3550
Aroller coaster car is traveling down a track at 22 m/s. the car has a mass of 2000 kg. what is the kinetic energy of the car? a) 22,000 j b) 968,000 j c) 484,000 j d) 44,000 j
Answers: 2

Chemistry, 22.06.2019 06:30, cadenhuggins2
Predict whether the changes in enthalpy, entropy, and free energy will be positive or negative for the boiling of water, and explain your predictions. how does temperature affect the spontaneity of this process?
Answers: 1

Chemistry, 22.06.2019 06:30, AleciaCassidy
Identify the missing numbers below to show the result of multiplying the numbers (1.6 × 10-19)(5.0 × 106) = c × 10d
Answers: 1
Do you know the correct answer?
125 grams of solid sodium hydroxide is combined with an excess of aqueous hydrochloric acid. The hea...
Questions in other subjects:

Biology, 15.12.2019 11:31



Social Studies, 15.12.2019 11:31


Mathematics, 15.12.2019 11:31

English, 15.12.2019 11:31


Biology, 15.12.2019 11:31

English, 15.12.2019 11:31




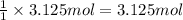 of water
of water