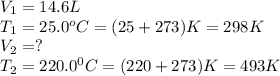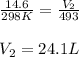Chemistry, 25.03.2020 18:00, littletiger4867
A sample of gas in a 14.6 L flexible container is at 25.0oC and 1.00atm. What is the volume of the sample when heated to 220.0oC and the pressure is constant? Remember that ALL TEMPERATURES MUST BE IN KELVIN! First: we add 273 convert Celsius to Kelvin so 25.0oC becomes 298 K We have constant pressure, two temperatures and one volume so we will solve for V2

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 08:30, breannaking9734
Which part of earth’s surface receives the most direct rays from the sun? a) equator b) ocean c) poles d) mountains
Answers: 2

Chemistry, 22.06.2019 14:20, greenbyron88
Which statement explains why the bonds between non metals tend to be covalent? the bonds are found to be nondirectional they have large differences in electronegativity they have small differences in electronegativity they have ions that produce an electrostatic pull
Answers: 1


Chemistry, 22.06.2019 22:00, robert7248
All of the following are homogeneous mixtures except a) sugar dissolved in water. b) orange juice. c) coffee with cream. d) household vinegar. e) apple juice
Answers: 1
Do you know the correct answer?
A sample of gas in a 14.6 L flexible container is at 25.0oC and 1.00atm. What is the volume of the s...
Questions in other subjects:



Chemistry, 12.03.2021 22:50

Mathematics, 12.03.2021 22:50


Mathematics, 12.03.2021 22:50


English, 12.03.2021 22:50



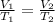
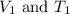 are the initial volume and temperature of the gas.
are the initial volume and temperature of the gas.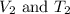 are the final volume and temperature of the gas.
are the final volume and temperature of the gas.