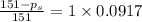Answers: 2
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 13:30, nasibamurodova
What are the chemical names of these compounds? ke: mg3n2: reset next
Answers: 1


Do you know the correct answer?
A solution contains naphthalene (C10H8) dissolved in hexane (C6H14) at a concentration of 13.06% nap...
Questions in other subjects:

Mathematics, 24.11.2020 19:40


Arts, 24.11.2020 19:40


History, 24.11.2020 19:40


Mathematics, 24.11.2020 19:40



Health, 24.11.2020 19:40


 is 137 torr
is 137 torr
 = relative lowering in vapor pressure
= relative lowering in vapor pressure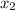 = mole fraction of solute =
= mole fraction of solute =