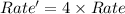Chemistry, 25.03.2020 07:01, mamas4539p79bw7
The reaction X + 2M → products has been found to have the rate law, rate = k[X] [M]2 . While holding the concentration of M constant, the concentration of X is increased from x to 4x. Predict by what factor the rate of reaction increases.

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 20:50, britotellerialuis
Evaluate this exponential expression,8. (2 + 3)2 – 42
Answers: 3

Chemistry, 22.06.2019 09:10, cheesedoodle
When a nucleus absorbs a neutron and then breaks apart, there are many products of the reaction. what is not a product of a nuclear fission reaction
Answers: 1
Do you know the correct answer?
The reaction X + 2M → products has been found to have the rate law, rate = k[X] [M]2 . While holding...
Questions in other subjects:

Mathematics, 01.03.2021 15:40

Mathematics, 01.03.2021 15:40

Computers and Technology, 01.03.2021 15:40

Mathematics, 01.03.2021 15:40



Biology, 01.03.2021 15:40

Mathematics, 01.03.2021 15:40

Social Studies, 01.03.2021 15:40


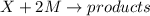
![Rate=k[X][M]^2](/tpl/images/0562/8462/02d02.png)
![Rate'=k[4X]^1[M]^2](/tpl/images/0562/8462/66381.png)
![Rate'=k[4]^1[X]^1[M]^2](/tpl/images/0562/8462/31177.png)