Chemistry, 25.03.2020 05:41, alexisthegirl
What volume of carbon dioxide will 3.26 g of antacid made of calcium carbonate produce at 37.0 °C and 1.00 atm in the stomach according to the following reaction?
CaCO₃ (s) + 2 HCl (aq) → CaCl₂ (aq) + H₂O (l) + CO₂ (g)

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 00:00, ashleyjaslin
Which type of bonding involves the complete transfer of a valence electron from a less electrogrative atom to a more electronegative one
Answers: 1

Chemistry, 22.06.2019 00:30, lasagnafoe
Used the balanced equation 2h2+ o2 - -> 2h2o. if you have 7.2 grams of o2 , how many grams of h2o can you produce ?
Answers: 2


Chemistry, 22.06.2019 19:00, Farhan54019
Which change to the system wood cause the freely-moving piston to lower?
Answers: 1
Do you know the correct answer?
What volume of carbon dioxide will 3.26 g of antacid made of calcium carbonate produce at 37.0 °C an...
Questions in other subjects:



Spanish, 01.11.2019 13:31


Chemistry, 01.11.2019 13:31



English, 01.11.2019 13:31

Mathematics, 01.11.2019 13:31


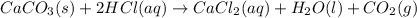
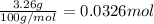
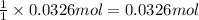 of carbon dioxide.
of carbon dioxide.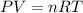 ( ideal gas equation )
( ideal gas equation )