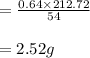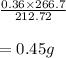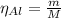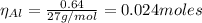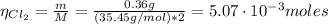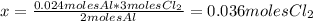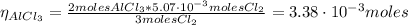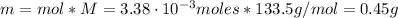Chemistry, 25.03.2020 05:47, ruchierosanp1n3qw
The balanced equation for the reaction of aluminum metal and chlorine gas is 2Al(s) + 3Cl2(g) → 2AlCl3(s) Assume that 0.64 g Al is mixed with 0.36 g Cl2. (a) What is the limiting reactant? Al Cl2 (b) What is the maximum amount of AlCl3, in grams, that can be produced?

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 04:00, nothingworksoutforme
The image shows a process that releases nuclear energy which statement best identifies the process shown the process must be fusion because energy is released the process must be fusion because of have your nucleus formed a smaller nuclei the process must be fission because a large nucleus breaks into smaller nuclei the process must be fission because neutrons are formed
Answers: 1

Chemistry, 22.06.2019 08:00, hdjsjfjruejchhehd
Define dew point. i am writing this part to be able to ask the question
Answers: 1

Chemistry, 22.06.2019 23:30, jade468
Substance a is a nonpolar liquid and has only dispersion forces among its constituent particles. substance b is also a nonpolar liquid and has about the same magnitude of dispersion forces among its constituent particles. when substance a and b are combined, they spontaneously mix.
Answers: 1

Do you know the correct answer?
The balanced equation for the reaction of aluminum metal and chlorine gas is 2Al(s) + 3Cl2(g) → 2AlC...
Questions in other subjects:


Mathematics, 14.10.2019 10:30

Mathematics, 14.10.2019 10:30

Mathematics, 14.10.2019 10:30





Arts, 14.10.2019 10:30