
Chemistry, 25.03.2020 05:51, kokokakahi
A mixture initially contains A, B, and C in the following concentrations: [A] = 0.600 M , [B] = 1.30 M , and [C] = 0.500 M . The following reaction occurs and equilibrium is established: A+2B⇌C At equilibrium, [A] = 0.410 M and [C] = 0.690 M . Calculate the value of the equilibrium constant, Kc.

Answers: 3
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 02:30, BornAdopted21
Which piece of equipment would me most useful for measuring the volume of some water? a. pan balance b. graduated cylinder c. tweezers d. flask quick
Answers: 2

Chemistry, 22.06.2019 06:30, Pizzapegasus1
Three cards with holes are arranged in a straight line. a light is shined through the first card’s hole and travels through all three cards. what does this tell you about light rays? a) that light is reflected b) that light is refractive c) that light travels in a straight line d) that light does not travel in a straight line
Answers: 1

Chemistry, 22.06.2019 10:30, kluckey3426
Asample of air with a volume of 2.20m3 at a pressure of 105 kpa and a temperature of 30c is cooled to 10c and the pressure is reduced to 75.0 kpa. what is the new volume? 6.9 1.34 2.56 43.0 2.88
Answers: 1
Do you know the correct answer?
A mixture initially contains A, B, and C in the following concentrations: [A] = 0.600 M , [B] = 1.30...
Questions in other subjects:

Biology, 03.02.2020 21:54

History, 03.02.2020 21:54

Mathematics, 03.02.2020 21:54


Mathematics, 03.02.2020 21:54



English, 03.02.2020 21:54

Mathematics, 03.02.2020 21:54

Mathematics, 03.02.2020 21:54


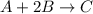
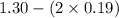
![K_{eq} = \frac{[C]}{[A][B]^{2}}](/tpl/images/0562/6350/666b5.png)
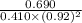
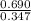
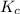 is 1.988.
is 1.988.



