
Chemistry, 25.03.2020 05:03, sajawalkhan148
The following questions refer to the gas-phase decomposition of ethylene chloride. Experiment shows that the decomposition is first order. The following data show kinetics information for this reaction: Time (s): 1.0, 2.0ln [C₂H₅Cl] (M): -1.625, -1.735 What was the initial concentration of the ethylene chloride?

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 20:30, mayamabjishovrvq9
1. calculate the approximate enthalpy of the reaction in joules. estimate that 1.0 ml of vinegar has the same thermal mass as 1.0 ml of water. iqnore the thermal mass of th sodium bicarbonate. note: it takes about 4.2 joules () to change 1.0 gram (1.0ml) of water 1.0 c
Answers: 2

Chemistry, 22.06.2019 02:00, issachickadi
Which of the following is not a good technique for managing used oil? a) have specific, labeled catch pans available for technicians who are collecting oil b) spills in your shop and any releases on pavement or outside should be poured down a drain c) do not use oil containers for antifreeze or other non-similar fluids d) be prepared for oil spills with the proper absorbents
Answers: 1

Chemistry, 22.06.2019 02:30, fordkenae
24 points and brainliest to anyone who can answer under 10 minutes with best ! the table below shows the role of different substances during photosynthesis. substance role during photosynthesis glucose stores chemical energy water combines with glucose to form carbon dioxide chlorophyll traps sunlight which of the following statements would correct one of the roles listed in the table? glucose combines with carbon to form water. chlorophyll reacts with light to produce carbon dioxide. water combines with carbon dioxide during photosynthesis. chlorophyll stores chemical energy needed for photosynthesis.
Answers: 1

Chemistry, 22.06.2019 12:00, angtrevv
In a laboratory, 1.55mg of an organic compound containing carbon, hydrogen, and oxygen is burned for analysis. this combustion resulted in the formation of 1.45mg of carbon dioxide and .89 mg of water. what is the empirical formula for this compound?
Answers: 1
Do you know the correct answer?
The following questions refer to the gas-phase decomposition of ethylene chloride. Experiment shows...
Questions in other subjects:

Mathematics, 16.12.2020 23:00


Mathematics, 16.12.2020 23:00

Mathematics, 16.12.2020 23:00


Mathematics, 16.12.2020 23:00

History, 16.12.2020 23:00


Mathematics, 16.12.2020 23:00

Health, 16.12.2020 23:00


![K=\frac{1}{t_2-t_1}\times \ln (\frac{[C_2H_5Cl]_{initial}}{[C_2H_5Cl]_{final}})](/tpl/images/0562/4390/3fd43.png)
![K=\frac{1}{t_2-t_1}\times (\ln [C_2H_5Cl]_{initial}-\ln [C_2H_5Cl]_{final})](/tpl/images/0562/4390/63342.png)
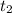 = final time = 2.0 s
= final time = 2.0 s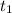 = initial time = 1.0 s
= initial time = 1.0 s![\ln [C_2H_5Cl]_{initial}](/tpl/images/0562/4390/01b3b.png) = initial concentration = -1.625 M
= initial concentration = -1.625 M![\ln [C_2H_5Cl]_{final}](/tpl/images/0562/4390/7c5a6.png) = final concentration = -1.735 M
= final concentration = -1.735 M![K=\frac{1}{2.0-1.0}\times [(-1.625)-(-1.735)]](/tpl/images/0562/4390/f39bc.png)
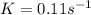
![0.11=\frac{1}{1-0}\times (\ln [C_2H_5Cl]_{initial}-(-1.625)]](/tpl/images/0562/4390/86ade.png)
![\ln [C_2H_5Cl]_{initial}=-1.515](/tpl/images/0562/4390/04037.png)
![[C_2H_5Cl]_{initial}=0.219M](/tpl/images/0562/4390/f9021.png)




