

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 01:00, baileysosmart
The diagram shows the positions of the sun, moon and earth during spring tides, when the high tides are at their highest and low tides at their lowest. what is it about these positions that causes these high and low tides?
Answers: 3

Chemistry, 22.06.2019 19:20, halledoll2002
Anyone who's in connections academy chemistry b have the factors that affect the rate of a reaction portfolio already done?
Answers: 3

Chemistry, 22.06.2019 21:00, lucyamine0
As we move from left to right across the periodic table, what is the general trend? a) atomic radii increase. b) electronegavitiy decreases. c) nuclear shielding increases. d) metallic character decreases.
Answers: 1

Do you know the correct answer?
Consider the following reaction: 2SO2(g)+O2(g)⇌2SO3(g) Kp=0.355 at 950 K A 2.75−L reaction vessel at...
Questions in other subjects:

English, 13.12.2019 21:31

Mathematics, 13.12.2019 21:31

Mathematics, 13.12.2019 21:31


Mathematics, 13.12.2019 21:31






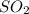 and
and 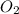
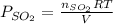
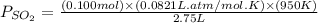
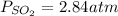
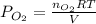
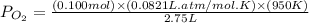
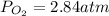
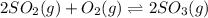
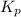 for above reaction follows:
for above reaction follows: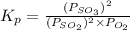
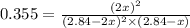
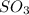 = (2x) = 2(0.664) = 1.33 atm
= (2x) = 2(0.664) = 1.33 atm




