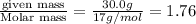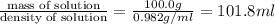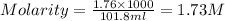Chemistry, 25.03.2020 03:47, haileywatkins
3. Calculate the molarity of an NH3 aqueous solution made up of 30.0 g of NH3 and 70.0 g of water at 25 °C. The density of water at that temperature is 0.982 g mL−1. M(NH3) = 17.034 g mol−1 A. 17.3 mol L−1 B. 24.7 mol L−1 C. 5.78 × 10−2 mol L−1 D. 0.578 mol L−1 E. 1.73 mol L−1

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 22:30, Aidanjsauer
Check the correct box to describe the periodic trends in electronegativity. electronegativity across a period: decreases. increases. electronegativity down a group: decreases. increases.
Answers: 2

Chemistry, 22.06.2019 09:00, kcarstensen59070
What type of energy do chemical bonds have? what type of energy is it converted to during chemical reactions? question 15 options: chemical bonds have kinetic energy, which is converted to potential energy during chemical reactions. chemical bonds have electric energy, which is converted to potential energy during chemical reactions. chemical bonds have heat energy, which is converted to kinetic energy during chemical reactions. chemical bonds have potential energy, which is converted to heat energy during chemical reactions.
Answers: 1

Do you know the correct answer?
3. Calculate the molarity of an NH3 aqueous solution made up of 30.0 g of NH3 and 70.0 g of water at...
Questions in other subjects:

Mathematics, 20.11.2020 22:30



Law, 20.11.2020 22:30

Mathematics, 20.11.2020 22:30



Mathematics, 20.11.2020 22:30

History, 20.11.2020 22:30


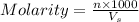
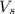 = volume of solution in ml
= volume of solution in ml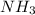 (solute) =
(solute) =