
A major component of gasoline is octane C8H18. When liquid octane is burned in air it reacts with oxygen O2 gas to produce carbon dioxide gas and water vapor. Calculate the moles of carbon dioxide produced by the reaction of 0.085mol of oxygen. Be sure your answer has a unit symbol, if necessary, and round it to 2 significant digits.

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 01:00, angelteddy033
Which of the following is not a true statement about dwarf planets? a the kuiper belt contains comets, asteroids, and dwarf planets. b ceres is a dwarf planet located in the kuiper belt. c the largest known dwarf planet in the solar system is named eris.
Answers: 2


Chemistry, 22.06.2019 10:40, yfgkeyonna
Ammonia and oxygen react to form nitrogen monoxide and water, like this: 4nh3 (g) + 5o2 (g) → 4no (g) + 6h2o (g) also, a chemist finds that at a certain temperature the equilibrium mixture of ammonia, oxygen, nitrogen monoxide, and water has the following composition: compound pressure at equilibrium nh3 65.1atm o2 31.3atm no 62.7atm h2o 65.8atm compound pressure at equilibrium nh3 65.3 atm o2 7.79 atm no 12.1 atm h2o 65.8 atm calculate the value of the equilibrium constant kp for this reaction. round your answer to 2 significant
Answers: 2

Chemistry, 22.06.2019 11:30, samantha9430
Determine the reaction and balance the following equations urgent due in the morning
Answers: 2
Do you know the correct answer?
A major component of gasoline is octane C8H18. When liquid octane is burned in air it reacts with ox...
Questions in other subjects:


Chemistry, 19.03.2020 00:49

Mathematics, 19.03.2020 00:49

Mathematics, 19.03.2020 00:49

English, 19.03.2020 00:49

Biology, 19.03.2020 00:49





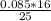 = 0.1 mole of CO₂.
= 0.1 mole of CO₂. 



