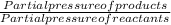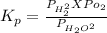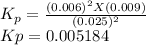The elementary reaction 2 H 2 O ( g ) − ⇀ ↽ − 2 H 2 ( g ) + O 2 ( g ) proceeds at a certain temperature until the partial pressures of H 2 O , H 2 , and O 2 reach 0.025 atm, 0.0060 atm, and 0.0090 atm, respectively at equilibrium. What is the value of the equilibrium constant at this temperature?

Answers: 3
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 02:00, lydiadmanautou04
Write a hypothesis that answers the lesson question, “while observing a chemical reaction, how can you tell which reactant is limiting? ” hypothesis: if a substance is the limiting reactant, then . . because . .
Answers: 1

Do you know the correct answer?
The elementary reaction 2 H 2 O ( g ) − ⇀ ↽ − 2 H 2 ( g ) + O 2 ( g ) proceeds at a certain temperat...
Questions in other subjects:

Mathematics, 13.02.2021 03:50


Social Studies, 13.02.2021 03:50

Mathematics, 13.02.2021 03:50

English, 13.02.2021 03:50




Mathematics, 13.02.2021 03:50

Mathematics, 13.02.2021 03:50