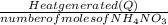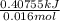Chemistry, 25.03.2020 02:03, AkramMasoud
Calculate the change in enthalpy for the reaction. (Use 1.0 g/mL as the density of the solution and 4.18 J/(g⋅∘C) as the specific heat capacity.) Express the enthalpy change in kilojoules per mole to two significant figures. ΔHrxn = nothing kJ/mol

Answers: 1
Other questions on the subject: Chemistry




Chemistry, 22.06.2019 04:30, homeschool0123
How many moles of air are there in a human lung with a volume of 2.4 l at stp? explain your answer
Answers: 1
Do you know the correct answer?
Calculate the change in enthalpy for the reaction. (Use 1.0 g/mL as the density of the solution and...
Questions in other subjects:

Chemistry, 16.07.2019 12:30


English, 16.07.2019 12:30

History, 16.07.2019 12:30


Chemistry, 16.07.2019 12:30

Social Studies, 16.07.2019 12:30

English, 16.07.2019 12:30


Mathematics, 16.07.2019 12:30


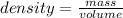
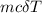
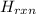 =
=