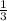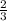Chemistry, 25.03.2020 01:21, FavvBella84
The total pressure for a mixture of N2O4 and NO2 is 1.5atm. If Kp = 7.1 at 25°C, calculate the partial pressure of each gas in the mixture. (HINT 1.5 = 2pNO2 + pN2O4) 2NO2 (g) = N2O4 (g)

Answers: 1
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 14:00, luisaareli6298
Calculate the energy required to ionize a hydrogen atom to an excited state where the electron is initially in the n = 5 energy level. report your answer in kilojoules
Answers: 1

Chemistry, 22.06.2019 19:50, strawberrymrmr756
Which sentence from holes contains an implied personality trait? stanley and his parents had tried to pretend that he was just going away to camp for a while, just like rich kids do. he'd just been in the wrong place at the wrong time. stanley felt somewhat dazed as the guard unlocked his handcuffs and led him off the bus. stanley nodded to show he understood
Answers: 3

Chemistry, 22.06.2019 20:30, jaydenbrock
Identify the correct mole ratio for each substance. sodium chloride (nacl) na: cl = 1: ammonium nitrate (nhno) h: o = 4:
Answers: 1
Do you know the correct answer?
The total pressure for a mixture of N2O4 and NO2 is 1.5atm. If Kp = 7.1 at 25°C, calculate the parti...
Questions in other subjects:

Physics, 05.05.2020 16:51



Mathematics, 05.05.2020 16:51




Mathematics, 05.05.2020 16:51


Biology, 05.05.2020 16:51


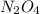 : 0.5 atm and
: 0.5 atm and 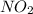 : 1 atm
: 1 atm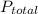 = 1.5 atm
= 1.5 atm