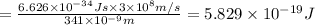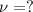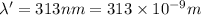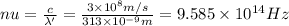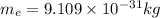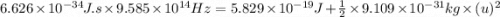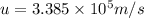The mathematical equation for studying the photoelectric effect is:
hν = W + 1/2 meμ^2 where ν is the frequency of light shining on the metal; W is the energy needed to remove an electron from the metal; and me and u are the mass and speed of the ejected electron, respectively. In an experiment, a student found that a maximum wavelength of 341 nm is needed to just dislodge electrons from a metal surface. Calculate the velocity (in m/s) of an ejected electron when the student employed light with a wavelength of 313 nm.

Answers: 2
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 08:30, breannaking9734
Which part of earth’s surface receives the most direct rays from the sun? a) equator b) ocean c) poles d) mountains
Answers: 2

Chemistry, 22.06.2019 16:00, bbrogle5154
If 15 drops of ethanol from a medical dropper weight 0.60g, how many drops does it takes from a dropper to dispense 1.0ml of ethanol? the density of ethanol is 0.80g/ml
Answers: 1

Chemistry, 22.06.2019 19:30, 2020sanchezyiczela
Draw the lewis structure for the trisulfur s3 molecule. be sure to include all resonance structures that satisfy the octet rule.
Answers: 3
Do you know the correct answer?
The mathematical equation for studying the photoelectric effect is:
hν = W + 1/2 meμ^2...
hν = W + 1/2 meμ^2...
Questions in other subjects:


Business, 22.11.2020 08:40


Mathematics, 22.11.2020 08:40

English, 22.11.2020 08:40


Mathematics, 22.11.2020 08:40

English, 22.11.2020 08:50

Spanish, 22.11.2020 08:50


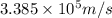 .
.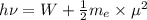
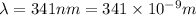
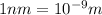
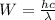 (Planck's equation)
(Planck's equation)