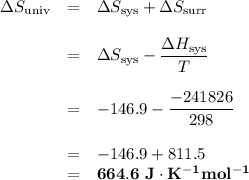Calculate the ΔH, ΔS and ΔSuniverse for this reaction.

Chemistry, 24.03.2020 23:01, Cartucho1978
½O2(g) + H2(g) ⇌ H2O(g)
Calculate the ΔH, ΔS and ΔSuniverse for this reaction.
Determine the spontaneity of the reaction.

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 05:50, ttangelique
What are the 4 phases of matter in order of increasing engery content?
Answers: 2


Chemistry, 22.06.2019 16:00, annsmith66
What statement goes against the kinetic theory of gases
Answers: 1

Chemistry, 22.06.2019 18:00, sandeebassett3
Mercury turns to vapor at 629.88 k how much heat is lost 175 g of mercury vapor at 650 current condenses to a liquid at 297 ca mercury turns to weber at 629.88 kelvin how much he is lost 175 g of mercury vapor and 650 coming condensers to liquidate 297 kevin
Answers: 2
Do you know the correct answer?
½O2(g) + H2(g) ⇌ H2O(g)
Calculate the ΔH, ΔS and ΔSuniverse for this reaction.
Calculate the ΔH, ΔS and ΔSuniverse for this reaction.
Questions in other subjects:



Business, 24.09.2020 18:01




Mathematics, 24.09.2020 18:01