Chemistry, 24.03.2020 23:12, ShelleyPSeliger
For the chemical equation SO 2 ( g ) + NO 2 ( g ) − ⇀ ↽ − SO 3 ( g ) + NO ( g ) the equilibrium constant at a certain temperature is 3.10 . At this temperature, calculate the number of moles of NO 2 ( g ) that must be added to 2.30 mol SO 2 ( g ) in order to form 1.00 mol SO 3 ( g ) at equilibrium.

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 08:00, flakko1899
An electron moved from shell n = 2 to shell n = 1. what most likely happened during the transition? a fraction of a photon was added. a photon of energy was absorbed. a fraction of a photon was removed. a photon of energy was released.
Answers: 1

Chemistry, 22.06.2019 13:00, nadiarose6345
In a copper wire, a temperature increase is the result of which of the following
Answers: 1


Chemistry, 22.06.2019 16:50, briansalazar17
Answer asap need by wednesday morning calculate the ph of 0.16m ch3cooh which has ka = 1.74 x 10-5 mol dm-3 best answer will be brainliest
Answers: 3
Do you know the correct answer?
For the chemical equation SO 2 ( g ) + NO 2 ( g ) − ⇀ ↽ − SO 3 ( g ) + NO ( g ) the equilibrium cons...
Questions in other subjects:











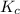 =
= ![\frac{[SO_{3} ][NO]}{[SO_{2}][NO_{2} ]}](/tpl/images/0561/8606/bc0fb.png)
![\frac{(1.00)(1.00)}{(2.30) [NO_{2} ]}](/tpl/images/0561/8606/3f85b.png) At equilibrium [SO₃] = [NO]
At equilibrium [SO₃] = [NO]