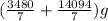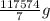Chemistry, 24.03.2020 22:30, 2023greenlanden
10 mol/h of 50 mol % NaOH is mixed with enough 6.5 mol % NaOH to produce a 10 mol % aqueous NaOH solution. The NaOH and water are initially at 25 OC. Assume the heat capacity of solution is 2.8 J/g C. How much of heat to be removed to keep the final solution at 10OC?

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 16:30, monnicawilliam
Energy is released during which phase changes? check all that apply. boiling condensing depositing freezing melting subliming
Answers: 2

Chemistry, 21.06.2019 23:30, ashleyjaslin
Calculate the expected ph values of the buffer systems from the experiments (a, b,c, d), using the henderson- hasselbalch equation, ph-pka+log[a-]/[ha]. use for pka values carbonic acid= 6.37, and acetic acid= 4.75.
Answers: 2

Chemistry, 22.06.2019 04:00, clairebear66
What three natural resources are found in the great lakes region
Answers: 2
Do you know the correct answer?
10 mol/h of 50 mol % NaOH is mixed with enough 6.5 mol % NaOH to produce a 10 mol % aqueous NaOH sol...
Questions in other subjects:




Mathematics, 09.05.2021 03:40

Mathematics, 09.05.2021 03:40


Mathematics, 09.05.2021 03:40

Mathematics, 09.05.2021 03:40

Physics, 09.05.2021 03:40


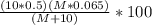
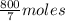
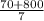
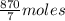
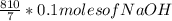
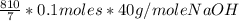
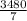 g of NaOH
g of NaOH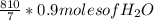
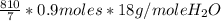
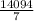 g of H₂O
g of H₂O