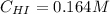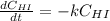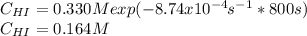Consider this reaction:
2HI(g) → H2(g)+ I2(g)
At a certain temperature it obeys t...

Consider this reaction:
2HI(g) → H2(g)+ I2(g)
At a certain temperature it obeys this rate law.
Rate= 8.74 x 10^-4 s^1
Suppose a vessel contains HI at a concentration of 0.330M. Calculate the concentration of HI in the vessel 800 seconds later. You may assume no other reaction is important. Round your answer to significant digit

Answers: 3
Other questions on the subject: Chemistry


Chemistry, 21.06.2019 22:30, colochaortiz20p7cajw
1.aluminum chloride (alcl3), and sodium hydroxide (naoh) can react to form aluminum hydroxide (al(oh)3) and sodium chloride (nacl). you have 13.4 g of aluminum chloride and 10.0 g of sodium hydroxide. answer the following questions: •what is the balanced equation for this reaction? •if you use all 13.4 g of aluminum chloride, how many grams of aluminum hydroxide can be formed? work must be shown to earn credit •if you use all 10.0 g of sodium hydroxide, how many grams of aluminum hydroxide can be formed? work must be shown to earn credit •how many grams of aluminum hydroxide will actually be made? which reagent is limiting? explain your answer.
Answers: 1

Chemistry, 22.06.2019 20:30, camerondillonn
Calculate the percent composition by mass of each element in al(oh)3. use at least three significant figures.
Answers: 1

Chemistry, 22.06.2019 22:30, itsmaddierae11
Which of the following molecules is polar? c3h7oh c2h5cooh
Answers: 1
Do you know the correct answer?
Questions in other subjects:

Mathematics, 06.11.2020 23:40

English, 06.11.2020 23:40

History, 06.11.2020 23:40

Mathematics, 06.11.2020 23:40






History, 06.11.2020 23:40