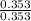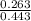A buffer solution is made that is 0.353 M in CH3COOH and 0.353 M in CH3COO- . (1) If Ka for CH3COOH is 1.80×10-5 , what is the pH of the buffer solution? (2) Write the net ionic equation for the reaction that occurs when 0.090 mol HI is added to 1.00 L of the buffer solution. Use H3O+ instead of H+ .

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 22:00, pettygirl13
Describe the particles of all three phases of matter in the root beer float. (how are the particles arranged and moving? )
Answers: 3

Chemistry, 22.06.2019 08:30, aydenmasonc
Which statement describes james chadwick’s discovery.
Answers: 2

Chemistry, 22.06.2019 14:00, JJlover1892
Ascientist measures the speed of sound in a monatomic gas to be 449 m/s at 20∘c. what is the molar mass of this gas?
Answers: 2
Do you know the correct answer?
A buffer solution is made that is 0.353 M in CH3COOH and 0.353 M in CH3COO- . (1) If Ka for CH3COOH...
Questions in other subjects:

History, 28.10.2019 07:31

Spanish, 28.10.2019 07:31

Mathematics, 28.10.2019 07:31




Biology, 28.10.2019 07:31


Geography, 28.10.2019 07:31


![\frac{[Salt]}{[Acid]}](/tpl/images/0561/5667/6743a.png)