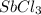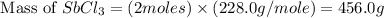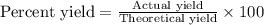Chemistry, 24.03.2020 20:06, brenda0014
Calcula el rendimiento de la reacción entre 2 mol de antonimo metálico(masa atomica 121.76 uma) y 5 mol de cloro(Cl2) (masa molar 70.90) para formar 444.6 G de tricloruro de antonimo (SbCl3) (masa molar 228.0 umal la reaccion quimica es la siguiente 4Sb+6Cl2 = 4SbCl3

Answers: 1
Other questions on the subject: Chemistry



Chemistry, 22.06.2019 09:10, cheesedoodle
When a nucleus absorbs a neutron and then breaks apart, there are many products of the reaction. what is not a product of a nuclear fission reaction
Answers: 1

Chemistry, 22.06.2019 10:30, cheyennecarrillo14
If you add 5.00 ml of 0.100 m sodium hydroxide to 50.0 ml of acetate buffer that is 0.100 m in both acetic acid and sodium acetate, what is the ph of the resulting solution? acetic acid: ka = 1.8. x 10-5
Answers: 1
Do you know the correct answer?
Calcula el rendimiento de la reacción entre 2 mol de antonimo metálico(masa atomica 121.76 uma) y 5...
Questions in other subjects:



Mathematics, 12.03.2020 21:25

Mathematics, 12.03.2020 21:25

Mathematics, 12.03.2020 21:26







 react with 6 mole of
react with 6 mole of 
 moles of
moles of