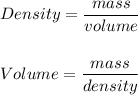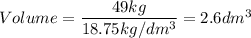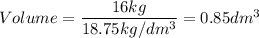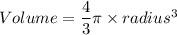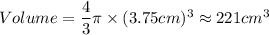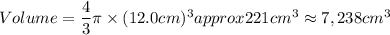Chemistry, 24.03.2020 19:58, AgarioEdit
A particular uranium alloy has a density of 18.75 g/cm3. Please answer the following questions below, providing the explanation to your answers. a. What volume is occupied by a critical mass of 49 kg of this alloy? b. The critical mass can be decreased to 16 kg if the alloy is surrounded by a layer of natural uranium (which acts as a neutron reflector). What is the volume of such smaller mass? Compare your answers to the approximate volumes of a baseball, a volleyball, and a basketball.

Answers: 1
Other questions on the subject: Chemistry



Chemistry, 22.06.2019 10:30, kylemartinez13
Which characteristics can be used to differentiate star systems? check all that apply.
Answers: 2
Do you know the correct answer?
A particular uranium alloy has a density of 18.75 g/cm3. Please answer the following questions below...
Questions in other subjects:



Mathematics, 24.08.2019 09:30



Biology, 24.08.2019 09:30


Social Studies, 24.08.2019 09:30


Mathematics, 24.08.2019 09:30