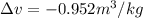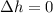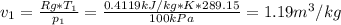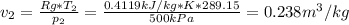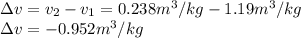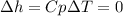Chemistry, 24.03.2020 20:12, 22chandlerlashley
Neon is compressed from 100 kPa and 16°C to 500 kPa in an isothermal compressor. Determine the change in the specific volume and specific enthalpy of neon caused by this compression. The gas constant of neon is R = 0.4119 kJ/kg·K, and the constant-pressure specific heat of neon is 1.0299 kJ/kg·K.

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 15:10, strodersage
Which statement describes the phase change that occurs when dry ice is placed in an open container at room temperature?
Answers: 1

Chemistry, 22.06.2019 18:00, jalenclarke25
What volume would 2.25 moles of ne has occupy at stp?
Answers: 1

Do you know the correct answer?
Neon is compressed from 100 kPa and 16°C to 500 kPa in an isothermal compressor. Determine the chang...
Questions in other subjects:

Mathematics, 27.04.2020 03:17

Mathematics, 27.04.2020 03:17

Mathematics, 27.04.2020 03:17

Mathematics, 27.04.2020 03:17

Health, 27.04.2020 03:17


Mathematics, 27.04.2020 03:17



Arts, 27.04.2020 03:17