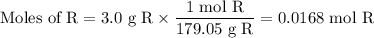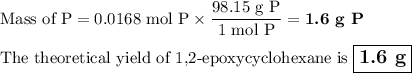Chemistry, 24.03.2020 20:15, soccerhannah290
The theoretical yield of 1,2-epoxycyclohexane is grams, when starting with 3.0 grams of trans-2-bromocyclohexanol. (Enter the number using 3 significant figures, i. e. 1.22) Given: 3.0 g of trans-2-bromocyclohexanol FW: 179.05 25 mL of 10% NaOH FW: 40 and density: 1.11 g/mL 1,2-epoxycyclohexane FW: 98.15

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 19:30, bryneosburn
What’s a special glass that most beakers are made of
Answers: 1

Chemistry, 22.06.2019 02:50, Jerrikasmith28
The conventional equilibrium constant expression (kc) for the system below is: 2icl(s) ⇄ i2(s) + cl2(g) [cl2] ([i2] + [cl2])/2[icl] [i2][cl2]/[icl]2 none of the listed answers are correct [i2][cl2]/2[icl]
Answers: 2

Chemistry, 22.06.2019 23:00, NewKidnewlessons
What is the energy in joules of a mole of photons associated with visible light of wavelength 486 nm?
Answers: 3

Chemistry, 23.06.2019 04:40, dd123984
Temperature is defined as a. the equivalent of heat. b. a measure of the average kinetic energy of the individual atoms or molecules composing a substance. c. how hot or cold it is. d. the total kinetic energy of the atoms or molecules composing a substance. e. none of the above is correct.
Answers: 1
Do you know the correct answer?
The theoretical yield of 1,2-epoxycyclohexane is grams, when starting with 3.0 grams of trans-2-bro...
Questions in other subjects:


Mathematics, 16.09.2019 17:30


Mathematics, 16.09.2019 17:30