

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 05:30, sethjohnson386pbnm3x
Modern weaponry has increased the number of deaths in wars and violent conflicts.
Answers: 3


Chemistry, 23.06.2019 02:00, FailingstudentXD
Calculate the molarity of each aqueous solution: a. 78.0 ml of 0.240 m naoh diluted to 0.250 l with water b. 38.5 ml of 1.2 m hno3 diluted to 0.130 l with water
Answers: 1

Chemistry, 23.06.2019 05:00, daytonalive6511
How many atomic mass units are equal to 1.672×10−24 g of protons?
Answers: 3
Do you know the correct answer?
2 H2(g) + S2(g) equilibrium reaction arrow 2 H2S(g) At a certain temperature, Kc = 1.30 ✕ 1010 for t...
Questions in other subjects:


Physics, 23.04.2020 16:17

Mathematics, 23.04.2020 16:18

Health, 23.04.2020 16:18


Mathematics, 23.04.2020 16:18





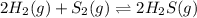
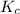 for above equation follows:
for above equation follows:![K_{c}=\frac{[H_2S]^2}{[H_2]^2[S_2]}](/tpl/images/0561/3034/d1522.png)
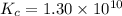
![[H_2]_{eq}=0.00400M](/tpl/images/0561/3034/ad07b.png)
![[S_2]_{eq}=0.00270M](/tpl/images/0561/3034/9642e.png)
![1.30\times 10^{10}=\frac{[H_2S]^2}{(0.00400)^2\times 0.00270}](/tpl/images/0561/3034/e2a23.png)
![[H_2S]_{eq}=23.7,-23.7](/tpl/images/0561/3034/68e27.png)





