
Chemistry, 24.03.2020 19:49, Lovelybunny321
Methylamine, CH3NH2, is a weak base that reacts according to the reaction CH3NH2 + H2O <--> CH3NH3+ + OH- The value of the ionization constant, Kb, is 5.25 x 10 –4. Methylamine reacts to form salts such as methylammonium nitrate, (CH3NH3+)(NO3-). a. Calculate the hydroxide ion concentration, [OH-] of a 0.125 molar aqueous solution of methylamine.

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 18:20, ineedhelp773
Which statement accurately describes the relationship between air pressure, air density, or altitude? as altitude increases, pressure increases. as altitude increases, air density increases. air pressure and density are lowest at sea level. denser air exerts more pressure than less dense air.
Answers: 2


Do you know the correct answer?
Methylamine, CH3NH2, is a weak base that reacts according to the reaction CH3NH2 + H2O <--> CH...
Questions in other subjects:


Mathematics, 24.03.2021 05:40

Mathematics, 24.03.2021 05:40

Mathematics, 24.03.2021 05:40

Physics, 24.03.2021 05:40


Mathematics, 24.03.2021 05:40




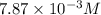
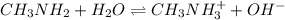
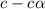
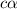
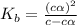
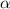 = ?
= ?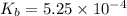
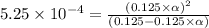
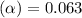
![[OH^-]=c\times \alpha](/tpl/images/0561/1862/0ea5e.png)
![[OH^-]=0.125\times 0.063=7.87\times 10^{-3}M](/tpl/images/0561/1862/08078.png)




