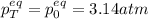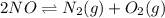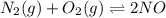Chemistry, 24.03.2020 19:22, brisamauro27
A mixture of 4.76 mol N 2 and 30.45 g NO is heated in a closed vessel to 2000 °C. After heating, the total pressure of the mixture at equilibrium is 3.14 atm . N 2 ( g ) + O 2 ( g ) − ⇀ ↽ − 2 NO ( g ) K p = 0.101 at 2000 ° C In which direction does the reaction proceed after heating to 2000 °C? The reaction is at equilibrium. The reaction proceeds toward the reactants. The reaction proceeds toward the products.

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 11:50, robert7248
The chemical bond connecting one nucleotide with the next one along the nucleic acid chain is called a
Answers: 3


Chemistry, 23.06.2019 00:00, savyblue1724707
How many moles of water are created if 3 moles of hydrogen react completely with excess oxygen?
Answers: 3

Chemistry, 23.06.2019 01:30, Michael845313
Ariver current has a velocity of 5km/h relative to the shore, and a boat moves in the same direction as the current at 5 km/h relative to the river. how can the velocity of the boat relative to the shore be calculated?
Answers: 1
Do you know the correct answer?
A mixture of 4.76 mol N 2 and 30.45 g NO is heated in a closed vessel to 2000 °C. After heating, the...
Questions in other subjects:

Social Studies, 17.02.2021 14:10

English, 17.02.2021 14:10

Mathematics, 17.02.2021 14:10

Mathematics, 17.02.2021 14:10


English, 17.02.2021 14:10

Social Studies, 17.02.2021 14:10