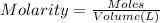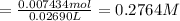Chemistry, 24.03.2020 19:14, roseemariehunter12
Initially a NaOH solution was standardized by titration with a sample of potassium hydrogenphthalate, KHC8H4O4, a monoprotic acid often used as a primary standard. A sample of pureKHC8H4O4 weighing 1.518 grams was dissolved in water and titrated with the NaOH solution. Toreach the equivalence point, 26.90 milliliters of base was required. Calculate the molarity of theNaOH solution. (Molecular weight: KHC8H4O4 = 204.2)

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 19:20, johnkings140
For a research project, a student decided to test the effect of the lead(ii) ion (pb2+) on the ability of salmon eggs to hatch. this ion was obtainable from the water‐soluble salt, lead(ii) nitrate, which the student decided to make by the following reaction. pbo(s) + 2 hno3(aq) → pb(no3)2(aq) + h2o losses of product for various reasons were expected, and a yield of 86.0% was expected. in order to have 5.00 g of product at this yield, how many grams of pbo should be reacted? (assume that sufficient nitric acid, hno3, would be used.)
Answers: 1

Chemistry, 22.06.2019 19:50, VoidedAngel
When the mercury level in a barometer decreases that atmospheric pressure has
Answers: 3

Chemistry, 22.06.2019 22:30, darceline1574
Vi limitens. vastery test select the correct answer. which statement explains why large atoms are more reactive than small atoms? a. large atoms have valence electrons farther from the nucleus and lose them more readily. b. large atoms have greater ionization energy, which they can utilize during a reaction. c. large atoms have a greater number of electrons that they can lose during a reaction. d. large atoms have more energy levels, so they have more energy to pass on in a reaction. reset next
Answers: 3

Chemistry, 23.06.2019 04:00, winterblanco
If you are told to get 100 ml of stock solution to use to prepare smaller size sample for an experiment, which piece of glassware would you use?
Answers: 1
Do you know the correct answer?
Initially a NaOH solution was standardized by titration with a sample of potassium hydrogenphthalate...
Questions in other subjects:

Chemistry, 03.04.2021 04:30

Mathematics, 03.04.2021 04:30





History, 03.04.2021 04:30


Mathematics, 03.04.2021 04:30

English, 03.04.2021 04:30


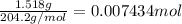
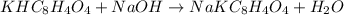
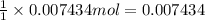 of NaOH
of NaOH