
Chemistry, 24.03.2020 19:17, DaylaReevaFEEVA5040
Tin(IV) sulfide, SnS 2 , a yellow pigment, can be produced using the following reaction. SnBr 4 ( aq ) + 2 Na 2 S ( aq ) ⟶ 4 NaBr ( aq ) + SnS 2 ( s ) Suppose a student adds 47.7 mL of a 0.474 M solution of SnBr 4 to 43.4 mL of a 0.179 M solution of Na 2 S . Identify the limiting reactant.

Answers: 1
Other questions on the subject: Chemistry



Chemistry, 23.06.2019 08:30, elijah4723
If you had to research a particular disease or area of concern in veterinary medicine and science, which one would you choose? why?
Answers: 1

Chemistry, 23.06.2019 10:10, nancysue1975
Calculate the h3o+ concentration in a solution of acetic acid if the concentration of molecular acetic acid present at equilibrium is 9.97x10-3 m and k for the dissociation is 1.86x10-5. ch3cooh(aq)+h2o(l)+> h3o+(aq)+ch3coo-(aq) show me how to get the answer.
Answers: 3
Do you know the correct answer?
Tin(IV) sulfide, SnS 2 , a yellow pigment, can be produced using the following reaction. SnBr 4 ( aq...
Questions in other subjects:


Engineering, 20.01.2021 05:30



Mathematics, 20.01.2021 05:30


Mathematics, 20.01.2021 05:30




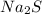 is the limiting reagent
is the limiting reagent 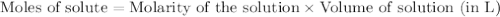 .....(1)
.....(1)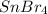 solution = 0.474 M
solution = 0.474 M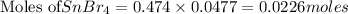

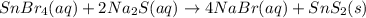
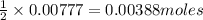 of
of 




