
A solution is prepared by adding 100 mL of 1.0 M HC₂H₃O₂(aq) to 100 mL of 1.0 M NaC₂H₃O₂(aq). The solution is stirred and its pH is measured to be 4.73. After 3 drops of 1.0 M HCl are added to the solution, the pH of the solution is measured and is still 4.73. Which of the following equations represents the chemical reaction that accounts for the fact that acid was added but there was no detectable change in pH?
(A)
(B)
(C)
(D)

Answers: 3
Other questions on the subject: Chemistry


Chemistry, 21.06.2019 20:50, karlyisaunicorn
Which real-world scenarios below represent physical and chemical changes? -running a car -exploding fireworks -mixing water and powdered drink mix -combining oil and vinegar to make salad dressing -taking aspirin for a headache -diluting bleach with water-digesting dinner-spreading peanut butter on bread
Answers: 2


Chemistry, 23.06.2019 01:00, breemills9552
What two factors can affect the properties of a hydrocarbon? a. the number of its carbon atoms and the number of single bonds b. the number of its carbon atoms and the arrangement of its atoms c. the arrangement of its atoms and the number of its double bonds
Answers: 1
Do you know the correct answer?
A solution is prepared by adding 100 mL of 1.0 M HC₂H₃O₂(aq) to 100 mL of 1.0 M NaC₂H₃O₂(aq). The so...
Questions in other subjects:



Physics, 30.06.2019 15:10


Mathematics, 30.06.2019 15:10



History, 30.06.2019 15:10


Mathematics, 30.06.2019 15:10


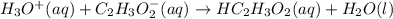 represents the chemical reaction that accounts for the fact that acid was added but there was no detectable change in pH.
represents the chemical reaction that accounts for the fact that acid was added but there was no detectable change in pH.



