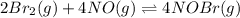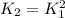Chemistry, 24.03.2020 17:51, rustalex6045
Consider the equilibrium reaction and its equilibrium constant expression. Br 2 ( g ) + 2 NO ( g ) − ⇀ ↽ − 2 NOBr ( g ) K = [ NOBr ] 2 [ Br 2 ] [ NO ] 2 For the reaction 2 Br 2 ( g ) + 4 NO ( g ) − ⇀ ↽ − 4 NOBr ( g ) select the equilibrium constant expression.

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 06:00, Sarahinator04
0.09 moles of sodium sulfate in 12 ml of solution
Answers: 3


Chemistry, 22.06.2019 09:20, nyceastcoast
Give the orbital configuration of the phosphorus (p) atom.
Answers: 1
Do you know the correct answer?
Consider the equilibrium reaction and its equilibrium constant expression. Br 2 ( g ) + 2 NO ( g ) −...
Questions in other subjects:

History, 18.03.2021 03:10

Computers and Technology, 18.03.2021 03:10




Mathematics, 18.03.2021 03:10

Health, 18.03.2021 03:10


Biology, 18.03.2021 03:10

Mathematics, 18.03.2021 03:10


![K_2=\frac{[NOBr]^4_{eq}}{[NO]^4_{eq}[Br]^2_{eq}}](/tpl/images/0560/9944/3b128.png)