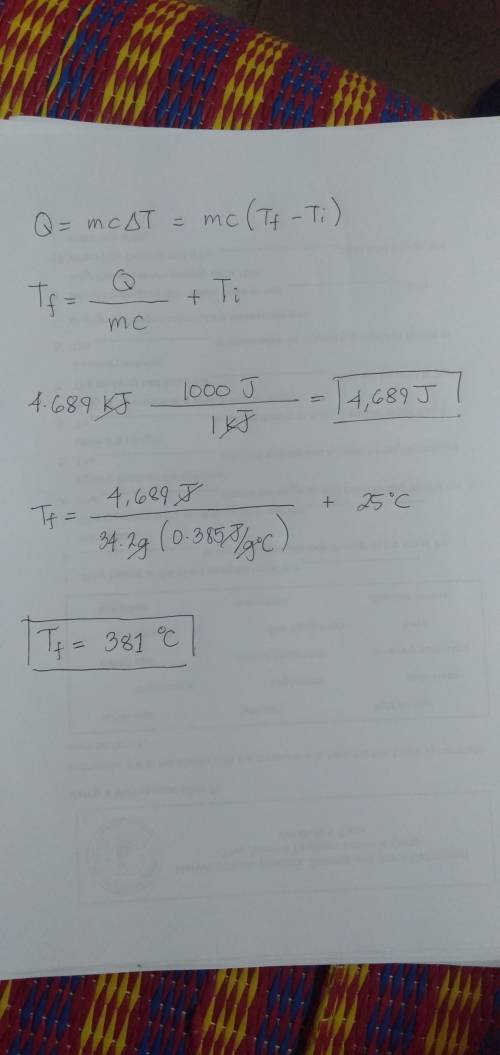Chemistry, 24.03.2020 14:03, paatnguyyen
The specific heat of copper is 0.385 J/(g °C). If 34.2 g of copper, initially at 25°C, absorbs 4.689 kJ, what will be the
final temperature of the copper?

Answers: 2
Other questions on the subject: Chemistry



Chemistry, 22.06.2019 22:30, lori90
Essay-alternative energy sources research sources of energy that are being developed. write a report of 350-400 words discussing the information you learned concerning the development of various energy sources and the impact that you think they will have on your life. include sources cited at the end of your report using the mla format. follow the rubric guidelines. note that wikipedia is not an appropriate resource for a research paper. worth 99
Answers: 3

Chemistry, 23.06.2019 00:30, natishtaylor1p8dirz
What is the chemical formula of magnesium bromide? a. mgbr2 b. mgbr c. mg2br2 d. mg2br
Answers: 3
Do you know the correct answer?
The specific heat of copper is 0.385 J/(g °C). If 34.2 g of copper, initially at 25°C, absorbs 4.689...
Questions in other subjects:

Mathematics, 04.04.2021 16:00

Health, 04.04.2021 16:00


History, 04.04.2021 16:00


English, 04.04.2021 16:00

Chemistry, 04.04.2021 16:00


Mathematics, 04.04.2021 16:00

English, 04.04.2021 16:00