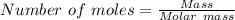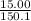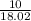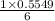Chemistry, 24.03.2020 05:29, lberman2005p77lfi
15.00 g of aluminum sulfide (150.1 g/mol) and 10.00 g of water (18.02 g/mol) react until the limiting reactant is used up. Calculate the mass of H2S (34.08 g/mol) that can be produced from these reactants. Notice that you will need to balance the reaction equation.
___Al2S3(s)+ ___H2O > ___Al(OH)3(s)+ ___H2S(g)
a. 13.89 g
b. 10.21 g
c. 19.67 gd. 9.456 g
e. 1.108 g

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 22:50, kanerobertrosss2213
At the current rate, a graph of carbon dioxide produced by fossil fuels over time would slope upward slope downward be horizontal be vertical
Answers: 3


Chemistry, 23.06.2019 01:00, kaykardash
Which polymers are most closely related? a. protein and nucleic acids b. cellulose and starch c. nucleic acids and starch d. nucleic acids and cellulose
Answers: 2

Do you know the correct answer?
15.00 g of aluminum sulfide (150.1 g/mol) and 10.00 g of water (18.02 g/mol) react until the limitin...
Questions in other subjects:

Mathematics, 18.05.2021 23:50






Arts, 18.05.2021 23:50

World Languages, 18.05.2021 23:50

Advanced Placement (AP), 18.05.2021 23:50