

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 08:30, melikefood01
Sally is making a model of a magnesium atom with an atomic mass number of 24 for her chemistry class. she has foam balls for the protons, neutrons, and electrons. she has added 6 neutrons to her model so far. how many more neutrons does she need to add to complete her neutral atom of magnesium?
Answers: 1

Chemistry, 22.06.2019 12:40, carebear60
Quiz1. which physical state of nitrogen has the highest entropy? a solid© b gasoc liquid
Answers: 1

Chemistry, 22.06.2019 14:30, darkghostmist
What type of reaction fuels the processes seen here?
Answers: 2

Chemistry, 22.06.2019 19:00, HaydenSturgis1
Which is the solubility product expression for caf2(s)?  [ca2+]/[f–]2  [ca2+][f2–]  [ca]+[f]2  [ca2+][f–]2
Answers: 3
Do you know the correct answer?
When calcium carbonate is heated, it produces calcium oxide and carbon dioxide. The equation for the...
Questions in other subjects:




Mathematics, 14.04.2020 07:26

History, 14.04.2020 07:26

History, 14.04.2020 07:26

Mathematics, 14.04.2020 07:26

Mathematics, 14.04.2020 07:26


History, 14.04.2020 07:26


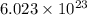 of particles and weighs equal to the molecular mass of the substance.
of particles and weighs equal to the molecular mass of the substance.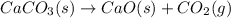
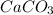
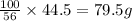 of
of 



